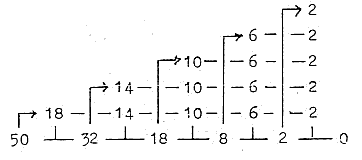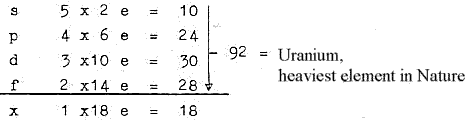Chemical elements - a 5-dimensional model applied in different sciences - |
|---|
|
Astronomy
- links:
|
Elements - links:
|
The Genetic Code -
links:
|
Language - links
|
|
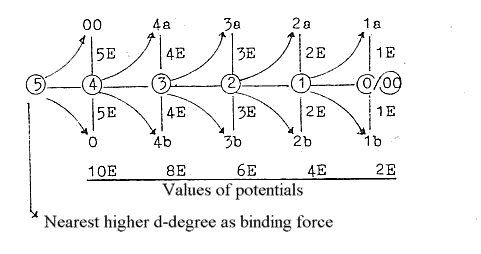
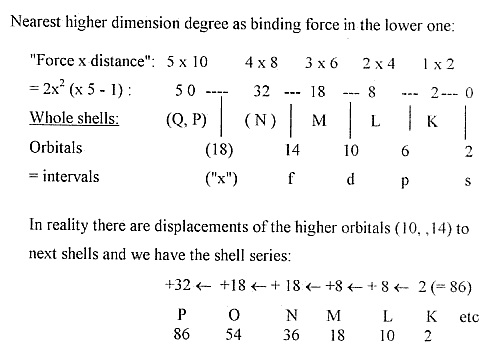
1. E-shells - orbitals from a dimension chain
|
|
|
|
|
©Åsa Wohlin
Free to distribute if the source is mentioned. Texts are mostly extractions from a booklet series, made publicly available in year 2000 |