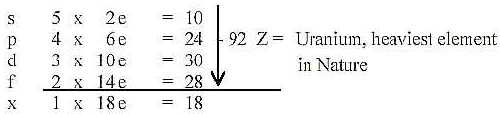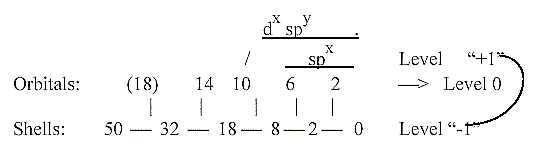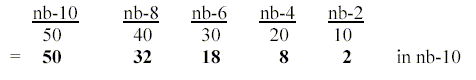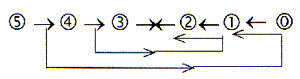1. The 2x2-series behind the
periodic system:
First to establishis the fact that a an elementary
number chain 2x2 , (x = 5 - 0) lies behind the
periodic system and electron configurations in atomic shells.
That is to say a version of the same kind as the
dimension model described in part Physics.
Whole shells and number of electrons in the
orbitals from the number chain 2x2
(x = 5-0), "theoretically":
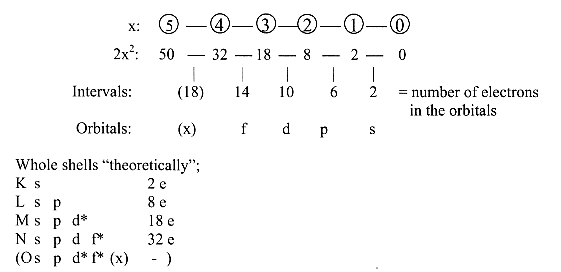
Orbital "x" here regarded as decomposed
in what has been called the "P"-and "Q"-shells".
*Through the displacement of d- and f-orbitals
to next shell, we get the real number series for whole shells:
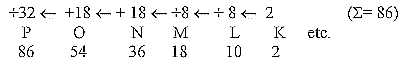
The 2x2 -series may also be derived as a chain
of energy, as Force times Distance in the dimension chain
of our model. We have defined higher dimension degree (d-degree)
in relation to a lower one as a binding force, the lower one
as potential with number value ("E") = sum of the
outer poles (or partial structures):
A dimension chain:
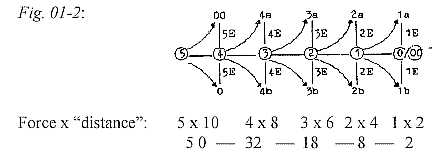
Counting on 5 orbitals,, although the 5th (x) not realized,
the theoretical maximum of Z becomes 110 Z, sum of the 2x2-chain.
As in the dimension model, we may regard
higher numbers transformed into lower ones. The whole series
of elements may be regarded as developed through one and the
same dimension chain, with secondary development in each step
giving a level chain: (This implies the aspect of "half-step"
displacements of the type "border to interval",
which in these papers has been suggested as an elementary
definition of a quantum jump.)
With numbers for the 2x2-chain as "borders":
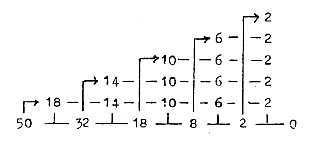 Fig. 01-3:
Fig. 01-3:
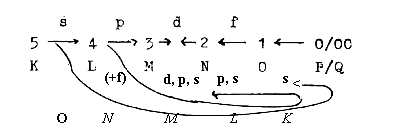
We get number of electrons theoretically in orbitals s,
p, d, f:
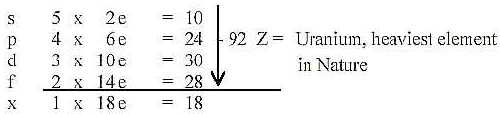
In reality a full second f-orbital of U is replaced by 4
s, 6 p, 1 d, 3 f electrons.
E-numbers and orbitals - sp(d)-hybridization:
Another way to derive the orbital numbers could be to
interpret them as sums of outer poles in lower degrees, in
agreement with the general postulates in our dimension model:
higher d-degrees polarized to outer poles in the lower d-degree.
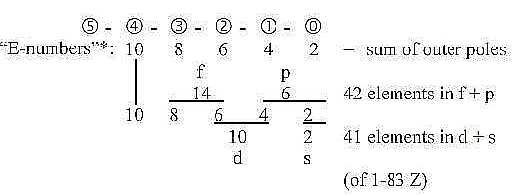
This interpretation may give aspects on sp(d)-hybridizations
in d-degree step (3)-2-1:
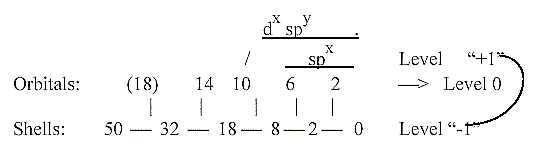
Such hybridizations, regarded as a superposed level, could
indicate that in the underlying scheme the whole shell numbers
may precede their differentiation into different orbitals.
*(As to this derivation of orbital numbers, see also paragraph
9.a).
There is still a third way to get the 2x<2-chain
from a simple number chain 5-4-3-2-1-0 which could be mentioned
here. It concerns transformations between number-base systems
(nb-x). (Such transformations give number relations in the
genetic code. See pdf-files on "The Genetic Code"
on the menu bar.)
Assuming the elementary number chain x 10
represents d-degrees connected with declining number-base
systems we have:
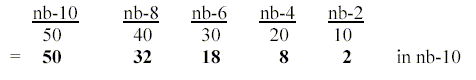
2. Lower steps or degrees in the number chain
as debranched from higher steps:
With respect to the orbital forms the s-orbital represents
the innermost orbital, the least polarized one but simultaneously
the last step in number of electrons in the chain. We could
here apply the loop model of a dimension chain, where debranched
d-degrees from higher d-degree steps meet "the other
way around":
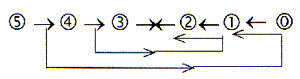
A sketch with question marks for the development of the f-orbitals:
The periodic system should be possible to regard from the double
aspect of polarizations and synthesis, where the direction of
polarization or disintegration on some underlying level is followed
by the synthezising direction (inwards) on a superposed level.
In this sense Uranium (the heaviest atom in
Nature) as a potential mass on a field level could be thought
of as an equal primary element as H, D, or He (1, 2, 4 A). (Cf.
perhaps the atomic radius of He and Fr (87 Z): both = 2,7 Å.)
Counterdirection "from outside" may be interpreted
as a condition for development of the higher orbitals. The
filling up of d-orbitals "demands" such counterdirection
from outside, expressed as s-electrons of the next higher
shell further out, and filling up of the f-orbital demands
both s- and p-electrons in still another shell further out.
Regarding a secondary centre
in step 3-2 and Pd:
Pd, 46 Z = ½ x max. Z in Nature, is the only element
where the d-orbital is "naked", the outmost one,
without any s-electron further out. It could represent a centre
in the 3-2-step, the step of the d-orbital number 10, where
counterdirections meet, perhaps a virtual way to level developments?
Or a channel in to the nucleus? Compare the geometrically
sensitive experiments with Pd for cold fusion?
Compare too that Z-numbers about 46 has
been identified as one kind of border in the periodic system
(between fusion and fission forces? (Gamow). See figures 07-2,
09-4.
To
3-4. Orbital
forms: Dimensional aspects on the geometries.
Quantum numbers
n, l, m, ms.
|

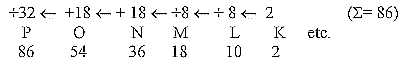

 Fig. 01-3:
Fig. 01-3: