|
Metals - non-Metals in relation to the octet rule:
This polarity concerns the relation to the octet rule and oxidation
numbers.
Most of non-metals are characterized by negative
oxidation numbers (in addition to positive ones with the exclusion
of oxygen (O) and fluorine (F) which have only negative ones). There
is H in the first s-orbital and the rest elements in the incomplete
p-orbitals. That's besides the inert gases without oxidation numbers.
The non-metals dominate first whole shells K,
L and M, get fewer in higher shells.
In these first two to three shells we find the structure building
elements of life, characterized by covalent bonds, which imply shared
lack with regard to the octet rule.
(With the loop model of the dimension chain in
mind, and the aspect of opposite directions in the chain, we could
regard these elements of life as representing the higher d-degrees
5-4-3. At the same time, as lightweight elements with Z-number in
lowest degrees, they should represent stages where more degrees
are debranched into motions, characterizing life.)
Negative oxidation numbers for L-shell elements C, N, O = -4,
-3, -2, (for H in K-shell -1), and for P, S in M-shell : -3,
-2. It seems as these numbers referred to d-degrees too, on a higher
molecular level.
All oxidation numbers, plus/minus, for instance
of carbon (C):
C: surplus of +4, in relation to a full K-shell (1s), surplus +2
in relation to full 1s and 2s in K+L-shell. deficit of -4 in relation
to full K-shell and L-shell s+p-orbitals = 10 e/Z.
Pauling's curve over the gradual change in electronegativity of
elements gets the S-form, where a "concave" part turns
to a "convex" part with a dividing inflection point. In
our model we have suggested such a polarity in geometrical terms
between poles from the polarization of d-degree 2: 2b = concave,
2a = convex. One expression for the polarity 0 < —> 00-poles
in lower d-degrees in terms of our model.
Pauling's curve defines also the ("gradual")
transition from covalent bonds to ion bonds between element.
In part Biochemistry in this booklet series, we deal with chemical
forces developed into a whole dimension chain on the superposed
molecular level, forces expressed in different bond types. We suggest
to interpret covalent bonds as expressions for the chemical force
4b (outward direction) in relation to metallic bonds 4a (inward
direction) which represent a shared surplus.* (All b- and a-poles
with features inherited from the first polarity 0-poles and 00-poles
respectively.)
To summarize: in these senses and on this level
we may regard the polarity non-Me / Me as referring to a centre
- anticentre or 0 / 00-relation (turned the other way around in
the 2x<2-series).
In each shell metals has a bigger circumference
than non-metals as anti-centra in relation tocentra. When ionized,
this relation is reversed.
*The ion bonds are interpreted as the force in
d-degree step 3-2, connected with the primary electromagnetic
polarity (+/-) as it is. Dipole bonds are interpreted as expressions
for the force in step 2-1 and van der Waal bonds (depending on
motions) as the force in step 1-0/00.
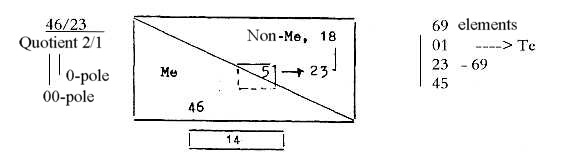
Number of metals and non-metals:
With the f-orbital elements excluded we have a
number relation about 2 / 1 among elements 1-83 (83 Z regarded as
the highest "stable" element.)
18 elements non-metals including 5 inert gases.
5 elements described as "transition
elements":
in used source Ge
32, As 33, Sn 50, Sb 51, Te 52 z.
46 elements metals in s-, p-, d-orbitals.
Fig. 07-1:
1-92 Z, division of Me and non-Me:
In the figure below a sketchy suggestion on how to look at it:
Sum of the 2x2-chain = 110, - 50
= 60
One orbital chain (18+14+10+6+2) = 50, - 18 = 32.... Sum 92.
Numbers for higher d-degrees as 50 and orbital
18 transformed into lower ones.
Fig. 07-2:
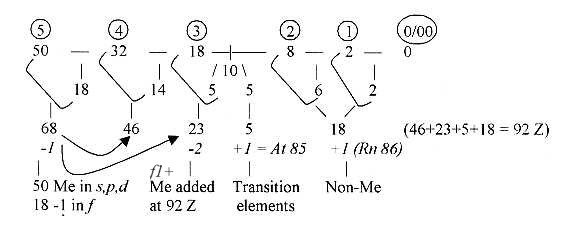
Of 50 Me: elements, in s+p-orbitals 18 +1, in
d-orbital 32 -1.
Z-sums of elements Non-Me without transition elements:
Non-metals ≈ 1/10 of elements
1 - 83 Z = 340 / 3486.
≈1/10 of elements 1 - 92 Z = 426 / 4278.
(In this relation we could eventually find one arithmetic expression
(1 - 10) for the 0- and 00-poles or b- and a-poles in our model.
(Very hypothetically a base for 10-power displacements in arithmetical
number derivations and operations.)
Z-sum of Me + Transition elements of 1-92 Z = = 3852.
Expressed in the triplet series: 3852 = 4 (432 + 321 + 210) = 12
x 321.
Cf. mirrored number 2583 = 21 x 123 = s+p+d-orbital sum 1-83
Z.
2583 = 21 x 123 (1-83 Z, s+p+d-orbitals)
3852 = 12 x 321 (1-92 Z, Me + Transition elements
in s+p+d+f-orbitals)
Could those mirrored factors reflect some turns both concerning
the f-orbitals versus s-p-d-orbitals and in the opposition Me- Non-Me
- and of additional non "stable" elements 82 - 92 Z? Cf.
50 - 32 - 8 - 8 - 2 - 0: 50+32 = 82, 2 + 8 = 10, opposite
part of the chain.
Different between factors: 21-12 = 9 and 321-12
= 198:
84 - 92 Z = 792 Z = 4 x 198 = difference between triplets
in the dimension chain:
"outwards" - "inwards": (543 + 432 + 321 + 210
) - (345 + 234 + 123 + 012).
*
To
8. Mass views: 1H
- 238U, lightest - heaviest element in nature,
9. Fusion and fission.
|