|
1. Temperature is a concept for motion
in microcosm, dimensionally dimension degree 0/00 in
this model:
When, with rising temperature in an atom
lattice, the oscillation amplitude of the particles exceeds
about 1/10 of the length of the bond between them, the bond
is breaking, so it´s said. This number relation could
eventually be thought of as originating from the last step
1→0/00 in the dimension
chain, read as numbers.
2. Factors in kinetic energy of gases dimensionally interpreted:
Formula:
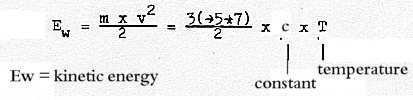
Interpretation of the d-degrees of motion:
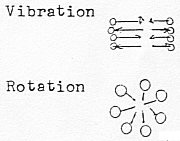
Vibration: = motions towards / from each other,
cccccccccccd-degree step 1
→ 1a/1b.
Rotation: = a) around own center c1 , b)
around bond center cccccccccccc2,
(2 planes) in d-degree step 2 →2a/2b
Translation: = rectilinear motions in 3 coordinates of space
cccccccccccc(path movements),
in d-degree step 3 → 3a/3b.
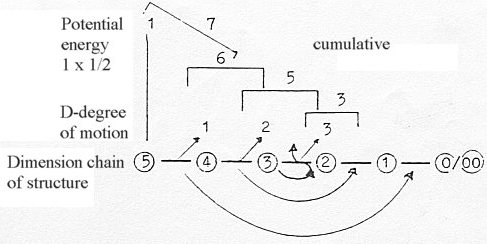
Motions of d-degree 1 - 2 - 3 in the dimension chain of
structure:
Energy Ew, numbers 7 - 5 - 3:
Monatomic gas: Translation:
3 x ½ k x T = 3/2 x k x T
Two-atomic gas, also: Rotation: +
2 x ½ k x T = 5/2 x k x T
Vibration:
+ 2 x ½ k x T = 7/2 x k x T
Hence, vibration and rotation only concern
two-atomic gases, while translation concerns monatomic gases
too. Two-atomic gases, with bound atoms, could be seen as
representing a higher d-degree.
- We can observe the increasing one-way direction
of the motion, from vibration to rotation to translation,
from the viewpoint of an individual coordinate axis,
this in accordance with the general aspects on the dimension
chain in this model, that multi-directions gradually "crystallize"
or are more precisely defined, that is more and more one-way
directed, towards lower d-degrees.
- Numbers 7-5-3: note numbers of a dimension
chain:
543 + 210 = 753.
A note about "micelles":
Concerning the relation vibration - rotation: compare so
called "micelles" in cell biology:
- Vibration gives a picture of micelles in laminar order,
an arrangement when density is high.
Vibration out of d-degree step 5 →
4, density as the physical quantity.
- Rotation gives the picture of micelles in circular order,
the arrangement when density is lower.
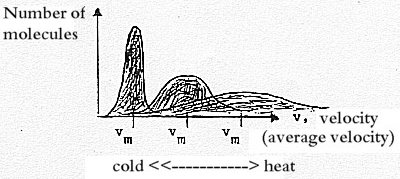
3. Temperature in relation to velocity:
Temperature has also been defined as
"a measure of the width of the spreading
of velocities"
of particles. (The quotation from a context concerning molecules.)
The higher temperature, the greater spreading
of velocities of the molecules. (Valid in volumes of lower
density at least. For gases, plasmas?)
(Inversely then the fusion scientists should
be able to get higher temperatures in their plasmas with
some kind of velocity spreader, shouldn't they? But fusion
should rather demand the opposite, the same velocity for
all nuclei, gathering them.)
Outer poles of temperature, extrapolated, would give v
max - v min in right angles towards
one another, the bar of molecules falling together with
the coordinate axes in the figure above.
Hence, cold and heat can be seen as perpendicular
quantities dimensionally.
The different aspects on temperature as velocity
spreading could perhaps elucidate the relation between the
physical quantities Temperature (in dimension degree 0/00)
and Density (in d-degree 4 with outer poles = 0 and 00),
and an inversion from radial to circular form between vector
fields in d-degree step 4→3
to 3-dimensional masses?
Temperature: degree numbers
- read in a dimension chain:
If just the division into degrees of the Celsius scale
should have any general validity, which seems absurd, it
should depend on the fact that water, H2O,
is central in life, and in the dimension chain: that it
"happens to be" just the atmospheric pressure
on the earth surface that gives level development to life,
which here is supposed to develop along a main axis of levels.
Numbers of H2O in a 2x2-chain,
x = 5-3-3-2-1-0:
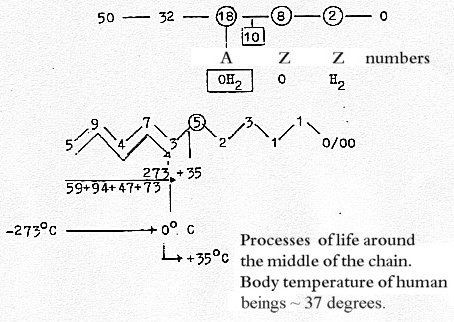
(An amusing thing: reading numbers in opposite direction
of that giving number 273, temperature interval in water
in solid phase, one gets 37+74+49+95 = 255. 255 Kelvin =
0°. Fahrenheit. But not the freezing point of water.)
According to earlier interpretation temperature
motions originate from d-degree steps 5→4,
4→3, 3→(2),
as from steps inwards from the other end of the chain, (3)← 2, 2← 1, 1←
0/00. One could then imagine that the development of temperature
went on through the 3-2-step "perpendicular" to
the chain towards superposed levels.
A couple of number operations:
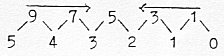 √
97/13 x 102 = 273,158 √
97/13 x 102 = 273,158
cccc _________
Cf. √(975 / 135),
x 102= 268,74.
Critical temperature of He = 268°
Boiling point of He = 269°...
|
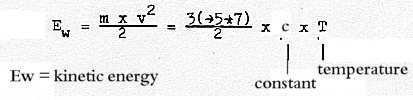




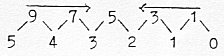 √
97/13 x 102 = 273,158
√
97/13 x 102 = 273,158