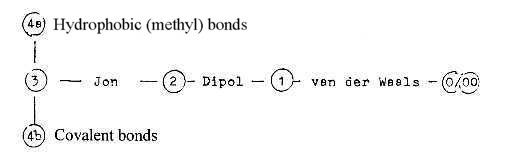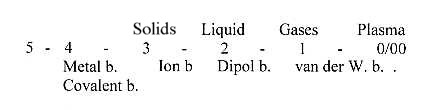The
chemists talk about 5-6 types of
chemical bonds, 5 within the organic
chemistry plus the "metal bonds"
within the inorganic one: -
Metal bonds - Covalent bonds
(electron pair bonds)
- Methyl
bonds (hydrophobic bonds)
-
Ion bonds
- Dipole bonds (hydrogen
bonds)
- van der Waals bonds
Is there anything besides
the number of them which indicates
that these type of bonds or chemical
"forces" in their schoolbook
formulations could correlate with
a dimension chain?
Any
such unambiguous identification
seems difficult to find (as when
it regards the known 4 forces of
physics). Yet, here some aspects
in that direction:
All
chemical bonds are said to be manifestations
of the electromagnetic force of
physics, perhaps while that is the
only force scientists have the feeling
they almost fully understand?
According to the
general postulates in our dimension
model underlying levels are to interpret
as binding forces on superposed
ones as higher d-degrees in relation
to lower ones. This implies that
all forces of elementary physics
should be involved as complex components
on the chemical level even if only
the electromagnetic one is mentioned.
Any closer explanation
of the hydrophobic bonds is not
given in here used sources, and
it's said that the covalent bonds
are not "fully explained"
either. Concerning the nuclear
force it was said some decades ago
that it seemed to include both gravitational,
inertial like components and electromagnetic
potentials. The theories and views
have changed since then, but still
it's said for instance that the
nuclear force may be totally analyzable
in spin-spin- and pathway dependencies.
From the viewpoint of our model
this just means that "forces"
as a concept for d-degree 4 are
translated into lower d-degrees
- into mass, charges and linear
relations and in a general sense
may be expected to appear in molecular
interactions.
Even
Mass becomes a force with this view. H+
and e- as elementary
particles on the underlying level,
become forces - and very central
ones - on the chemical level and
should be identified and regarded
as such, mediators as they are in
most chemical bonds. They become
counterparts to π-mesons
and other quanta of forces which
the physicists call "carriers
of forces". In physics
the distinction is made between
polar and non polar forces, between
the electromagnetic force and the
weak force as polar ones, gravitation
and nuclear force as non polar The
same contradiction appears among
the chemical bonds:
Hydrophobic
bonds become of the type +/+ between
CH3-groups for instance, where the
"plus sides" of the H-atoms
are turned towards each other: that's
"methyl bonds".
Covalent
bonds are of the -/- type, electron
pairing as essential feature. Hence,
both these types are non polar from
the viewpoint of charge.
On
the other hand ion, dipole and van
der Waals bonds are all three polar,
built upon the attraction between
opposite charges.
Here
a first sketched suggestion of how
to look at relations to the dimension
chain: 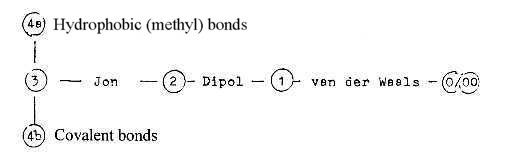
Hydrophobic← →Covalent
bonds (4a --- 4b):
It
should be underscored that none
of these bonds are fully understood
which may imply that other forces
than the electromagnetic one (EM)
are involved. In the model here
we have assumed the EM-force developing
first in step 3→2
in a dimension chain of forces (see
that
file in Physics). As
(-/-)-bonds the covalent electron
pairing bonds may be regarded as
complementary to the the nuclear
force between protons in the atomic
nucleus, which translated to the
superposed molecular level gets
expressed in hydrophobic bonds,
type (+/+). Compare the covalent
bonds, "explained" with
the octet rule on one hand (more
about this below) and on the other
the number 8 in the nuclear force:
according to older views on this
strong force one could find three
times 8-9 potentials in it. And
the strength of that force is roughly
said developed first at 8 Z (with
about 7,6 MeV per nucleon.) Even
if maximum is reached first at 26-28
Z.
(Are there
eventually virtual positrons in
the electron pairing bonds, as one
have speculated about virtual anti-protons
in the depth of atomic nuclei? Virtual
positrons out of the negative energy
of empty space?
 If
so it would be in accordance with
the here adopted general aspect:
the stepwise building-in of the
opposite pole, the 00-pole, on different
levels.) The relation between
the two types of bonds becomes that
between outward and inward directions,
from 0-pole and 00-pole respectively:
the covalent bonds outwards the
surface, the electron shells, and
the hydrophobic bonds inwards, analogous
to protons inwards the atomic nuclei.
(Hydrophobic
bonds may also be regarded as consequence
of the covalent ones, developed
from them, as there is always an
anticenter (~ a 00-pole) for a unit
as 0-pole, in the same sense that
the 00-pole represents the end of
a haploid dimension chain in relation
to the 0-pole as start, and represents
lower d-degrees and levels in relation
to higher ones.) Both types
of bonds may be characterized as
mutually counterdirected, cf. d-degree
4 as double direction, while bonds
of the ion and dipole types gets
rectified: 
The
electron pairs in the covalent bonds
have opposite = counterdirected
spin. Cf. double direction in d-degree
4 and increasing unidirection towards
lower d-degrees according general
views in our model. Hence,
in these both forces or bonds we
find features of the "FA"
and "FG"-forces
from the physical level, FA
as the outward acceleration force,
FG as
Gravitation. General definitions
in the dimension model say that
the 0-pole represents first binding,
integrating force, the 00-pole the
primary polarizing one, but in d-degree
3 turned to circular geometry becomes
an "aggregating" one.
It's easy to state as a fact that
covalent bonds are integrating,
the outward opposite directions
leading to bonds building bigger
and bigger molecules and increasing
order towards superposed levels.
It's the foundation of life chemistry,
the shared lack of completeness.
How a hydrophobic
"force" primarily may
act as polarizing in a cell, is
here left as an open question. (Maybe
the methylation, marking inward
direction, of positions in genes
should be studied from this aspect?)
Yet, like gravitation it appears
as aggregating on a weaker, superposed
level.
The results of
these forces could be seen as expressed
in next step, the poles of d-degree
3, proposed as radial versus circular
in elementary geometrical terms:
The covalent bonds may be regarded
as radial in their main valences.
The hydrophobic bonds are described
by chemists as if they had "circular
structure", that's with feature
from the 00-pole, the anticentre
pole. They are demarcating
a "sphere" within which
water is driven out; the non polar
methyl groups repelling the polar
water molecules. (One may after
all ask why?)
The
hydrophobic bonds are also central
for the building of cell membranes
- a "circular" structure,
shell creating, related to d-degree
2 of surfaces.
Cell
membranes could perhaps be regarded
as expression for the nuclear force
on this superposed level, (inverted
to anticenter or expanded). Mass ←→Space,
the complementary concepts regarded
as the poles in terms of physical
entities in d-degree 3 in our model
can also be one aspect on the relation
between hydrophobic and covalent
bonds: Mass is on the atomic level
connected with the nuclei and protons
(H) and may as an expression for
gravitation appear in the hydrophobic
aggregations which have got the
name of "bonds".
The
covalent bond are built on electrons,
most closely related to Vacant Space
and outward acceleration, the complementary
force to gravitation (see interpretations
in Physics). The covalent bonds
may be regarded as the essential
space-building ones.
Covalent bonds - some more aspects:
Covalent
bound molecules are more mobile
than metals and salts. In outward
direction the number of motion moments
increases towards lower d-degrees
and higher levels. And the level
development - eventually through
counterdirection from other units
outside - gives increasing degrees
of freedom (as for instance in the
OH-groups of carbohydrates). Life
as motion.
The
covalent bonds develop outwards
toward lower d-degrees as in -4→3→
2→1-steps
in several ways: - Molecules
get ionized - that is polarized
in charge, a kind of parallel to
the ion bonds in what could be seen
as a secondary developed d-degree
chain within covalent bonds. -
The bonds get differentiated into
main valences and side bonds, in
∑- and
π-bonds,
a structural relation of 180°
to 90° between overlapping orbits
which implies coordinate axes corresponding
to a d-degree step 4 →3
in a dimension chain interpreted
in terms of angle steps. -
The development from sp3 to sp2-hybridization
(as in diamond in relation to graphite),
from 4 bonds around a C-atom to
3 with delocalization of 1 electron,
implies a step from 3-dimensional
spherical structures to 2-dimensional
plane ones: a step towards lower
d-degrees which also implies more
motion moments. 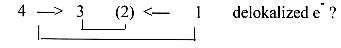
(The
step from sp3- to sp2-hybridization
has been regarded as a condition
for life.) The covalent bonds
become increasingly unidirected
- as through angle steps - towards
lower valence numbers as in the
atomic sequence C →
N →
O with valences 4 - 3 - 2. The molecules
get more and more of a dipole character,
a development which becomes a condition
for the dipole bonds.
Ion, Dipole, van der Waals bonds:
These bonds are explained
by the electromagnetic force between
charges plus and minus but a condition
for the ion bonds is the octet rule
behind the covalent bonds which
isn't explained by this electromagnetic
force. Ion bonds (3 --
2):
There is a gradual
changeover between covalent
bonds and ion bonds
according the Pauling's curve for
the electronegativity of atoms,
between bonds of dominant covalent
character and those of dominant
ion character. This curve has however
an inflexion point which may be
interpreted as a border, representing
a structural change of dimensional
character. (Cf. a sinus curve as
projection of a rotating vector
in a unity circle: 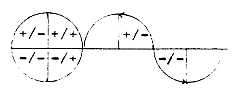
Ion
bonds have traits of both the metal
bonds and the covalent ones, both
inward and outward directions. (Cf.
poles 4a--4b of d-degree 3.)
In typical ion
bonds as salt crystals for instance
the structure building extension
is principally unlimited as in metals,
in opposition to the "individual
level" of covalent bound molecules
and differentiation in structures.
In this sense the ion bonds are
characterized by continuum in relation
to the feature of quantum jumps
of covalent bonds - an opposition
continuum - quantified which we
have seen as one aspect on the poles
0 and 00 (part Physics).
However,
the ion bonds in opposition to metal
bonds are quantified in steps +/-/+/-/+/-.
polarized in charge. But the transition
of electrons from metals to non
metals, creating the charged ions,
is totally dependant on the octet
rule. In which sense 3-2-dimensional?
Ion bonds build 3-dimensional regular
space structures, volumes as in
salt crystals, and grows "spherically",
with addition of layers (d-degree
2). Polarizations of Charge:
With the model of a dimension chain through polarizations it becomes
natural that also charge as property and physical quantity gets
polarized further from units of +/-. On the atomic level the d-orbital
for instance have been illustrated as with single electrons divided
on the half axis around the origin in the coordinate system. With
polarizations as the basis for distribution of charge this should
principally be quantified and possible to express as a series of
the type e →1/2 e →1/4
e… or e2 →e →
√e, with the electron as a wave
function or the like and secondarily still more complex divisions
on a molecular level. (See further files Chemical
Elements.)
"Activation"
of molecules within biology may
be assumed as more or less partial
displacements of charge - as partial
or "half steps" from border
to interval or the inverse, also
here regarded as the basis for the
concept discontinuity.
In
ion bonds one can talk about whole
charge transitions, in dipole bonds
of half or partial ones as of the
H-atoms in H-bridges. Hence, in
a step from ion bonds to dipole
bonds we have a polarization step
outwards in the dimension chain.
Chemists talk
about "delta charges"
in connection with dipole
bonds.
Dipole
and van der Waals bonds (2 → 1 → 0/00): The
dipole bonds may be regarded as
developed from the covalent bonds
when small molecules become more
and more polar towards lower valence
numbers of atoms as C, N, O (suggested
to be read as d-degrees).
While
ion bonds create solid volumes of
aggregation, the H-bridges are unidirectional
and linear and bind molecular chains
to one another, laterally as in
the H-bridges of DNA and between
protein chains, creating "ladder"
structures and layers.
Hence,
they can be connected with d-degree
step 2 - 1, also with regard to
the structures they create.
The dipole bonds
between H2O-molecules
in water render the water molecules
the flat form - a reason to identify
the phase of liquids as of d-degree
2. Distance and Time
This pair of concepts may be applied
to the dipole bonds in relation
to the van der Waals bonds. -
Dipole bonds,
out of unsymmetrical
distribution of charge in space,
linearly:
entity: Distance
- van der Waals bonds,
out of "unsymmetrical"
distribution of charge in Time In
dimension steps:
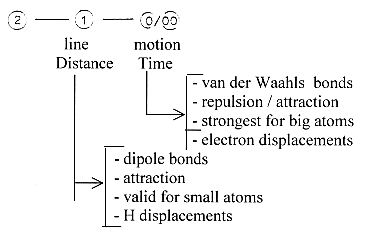
van der Waals bonds:
These bonds depend on the rotation
of the electrons, derive from "induced
dipoles" or "temporary
dipoles", represent time-dependant
couplings.
The
poles 1a and 1b of d-degree 0/00
in our dimension model have been
defined as "motions towards
each other" and "motions
from each other" respectively.
Expressed in terms of charge that's
attraction and repulsion. Both moments
are included and alternate in van
der Waals bonds. The bond
as a "line" between the
atoms is polarized in these motions
to and fro as in a dimension step
1 →1a/1b.
Change of signs are also included
in this type of bonds according
to the chemists. Compare the description
in our model: motions towards each
other, derived ultimately from inward
direction in d-degree 4, defines
a new centre, a new 0-pole and therewith
outward direction. While "motions
from each other", ultimately
derived from the 00-pole and outward
direction in d-degree 4, defines
a new anticentre and therewith new
inward direction. This implies a
sign exchange as direction change
in terms of the dimension model. The
van der Waals force is generally
prevalent between atoms and molecules
close to each other. Hence, in this
force an aggregating multidirectional
force seems to turn up again and
it would perhaps be possible to
identify a gravitational component
of inward direction in this force.
Primary poles of d-degree 4 "meet
again" in d-degree 0/00, which
makes it reasonable that the force
of d-degree 4a (inward direction
and the pole of manyfoldness) reappears
at the end of the chain of charge
polarizations.
Like
the gravitational force in microcosm,
the van der Waals force is also
very weak.
With the assumption
that all forces on the physical
level should reappear in some form
on the biochemical one, the question
may arise how the weak interaction
could manifest itself in biochemistry?
May it be found somewhere in the
development of the covalent bonds
towards biological levels, perhaps
as interaction between levels? Or
as a factor in the spiralling of
nucleotides and proteins? Or just
as one cause for the curious fact
that proteins are broken down and
have to be built up again all the
time? (This some vague speculations.)
The octet rule:
Covalent
bonds depend on what is called the
"octet rule" and are usually
explained as the "endeavour"
of the atoms to reach filled s-
and p-orbitals towards the
environment, 8 electrons as the
border for a whole shell.
This
octet rule goes beyond what is explained
by the electromagnetic force which
implies balance between protons
in the nucleus of an atom and number
of electrons in the shell.
With
a more general aspect we could imagine
the atom alternate between two roles,
as an entirety in itself, a relative
5-dimensional whole, in proton-electron-balance,
and its other role as part in the
entirety, a 0-pole in relation to
its surroundings. This in the same
way as human beings alternate between
states of sleep, closed in themselves
as entireties, and states of being
awake, the role of being parts in
relation to the surrounding world. Number
8 - some interpretations from the
viewpoint of our dimension model:
- In the 2x2-series
behind the periodic system from
which whole shells and orbitals
can be derived, we have number 8
in d-degree 2. 
D-degree 2 represent surfaces, the
demarcation of a unit from the surrounding,
a shell.
- In the 2x-series,
which hypothetically has been assumed
valid in direction inwards, we have
after 3 polarizations (from 0/00)
number 8 at d-degree 3
- In
a 3-dimensional coordinate system
there are 8 space quadrants: The
number 8 could be traced back to
the 3rd d-degree of space.
(The coordinate
system is also defined through 6
outer poles, and with the polarity
"origin versus anticentre",
represented by the s-orbital, added
it gives number 8.) - In angle
steps 360°→180°
→
90° →
45°, a circle is divided in
8 parts. 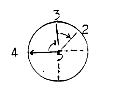
- Another aspect: In our
model we have assumed that Charge
as such is a property (the physical
quantity) defined in d-degree 2
in relation to mass analysed as
3-dimensional. Here at d-degree
2 this property of Charge would
possibly appear as still unpolarized.
In this sense "complete". -
In a dimension chain the outer poles
of d-degree 2 is 3a and 3b. Compare
perhaps what becomes the 6 electrons
divided in 3 + 3 with opposite spin
in the p-orbital. 
- Outer poles of d-degree
3 is 4a 4b, representing the opposite
vectors of d-degree 4 on an underlying
level. Sum of poles = 8. In this
way we can trace the octet rule
back to d-degree step 4→
3 according to our suggested views
on covalent bonds above. -
The "loop version" of
our dimension model implies that
orbitals in steps
0/00-1 and
1-2 (s and p orbitals)
are regarded as debranched from
higher steps outwards, and the opposite
directions in the chain meeting
in step 3--2:
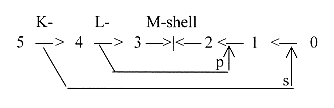
We
have s- and p-orbitals
as debranched from inner shells
and remember that H belongs to the
K-shell, C, N and O belong to the
L-shell, P and S to the M-shell.
(More about the
octet rule in files Chemical
Elements.)
Metal bonds - in inorganic chemistry:
How
interpret the inorganic metal bonds
in this suggested scheme?
a.
They may be regarded as the collective
correspondence to an individual
atom with protons assembled in the
centre, electrons "displaced"
to a shell. Delocalized electrons
described as uniting "clouds"
around the positively charged metal
ions.
The
polarity individual - collective
has the feature of 0- and 00-poles,
defining d-degree 4.
Generally
the 00-pole represent manyfoldness
and anticentre in relation to centre.
It's the source for inward direction.
This vector aspect could be one
factor in the metal bonds where
the atoms get closer to one another:
the ionizations and delocalization
of outer electrons as a means or
a result.
The common cloud of electrons is
said to act "uniting"
on a collective atomic level, but
such an explanation is hardly convincing.
Why do the electrons get delocalized
- and in which sense do they appear
as clouds? Is there really reason
to interpret the metal bonds as
purely an expression of the electromagnetic
force?
b. Metals dominate
more and more toward heavier elements
and all elements in higher orbitals
as d and f . In atoms
Mass is concentrated to the nuclei
and Mass is connected with gravitation,
the FG-force;
electrons in the shell are closest
to Vacant Space, connected with
the FA-force.
We could in this respect connect
the metal bonds with the gravitational
force. The growing number of neutrons
in heavier elements emphasizes also
the Mass property of nuclei. Hence,
in metal bonds one factor could
be an example of Mass as binding
force in relation to lower d-degrees
as Charge, interpreted as of d-degree
2, a factor which should be part
of the interpretation. c.
Metal bonds have similarities with
other bonds as if they represented
the collective complementary poles
to bonds developed from individual
atoms:
- With hydrophobic
bonds, in its non polar type, its
aggregation of similar atoms with
a relative surplus of electrons
and in the gathering of plus-charges
and "driving out" of electrons,
as hydrophobic bonds drive out water.
But the hydrophobic bonds develop
from covalent bonds on an individual
level.
- With ion bonds
in building solid structures and
in the aggregating type, characterized
by continuum like ion bonds in salts
but in opposition to these not quantified
with respect to charge. Here the
ion bond has the individualized
crystal structure of +/-variations,
possible to regard as radial in
relation to metallic bonds with
their "spherical" (?)
clouds. (Poles of d-degree 3.)
- With dipole bonds in the
character of layers which the delocalized
electrons give metals, surface layers
that may glide over one another.
A corresponding form in the development
of individual covalent bonds is
what is explained through the term
sp2-hybridizations, with
"delocalized" common electrons
giving plane molecular structures.
Again we
remind of the coupling between d-degree
step 4→3
and the step 2 → 1
in the loop version of a dimension
chain in our model. 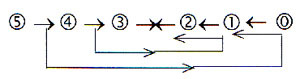
-
Finally, there are a certain relationship
with the plasma phase in the mobility
of the delocalized electrons. With
the plasma phase as the ultimate
separation of protons and electrons
interpreted as the phase in d-degree
0/00, we get the connection between
the outer poles of d-degree 4, 0
and 00, combined in last d-degree"
0/00" of motions.
In the "haploid"
version of a dimension chain the
pole 0 represent 5 and pole 00 the
d-degree 0/00.
Hence,
we could in a certain sense regard
the development of phases and types
of chemical bonds as occurring between
the individual and covalent type
outwards and the metal bonds inwards.
Some kind or kinds
of metals should be an essential
factor in the development of life
structures - as they surely are!.
Connections between
bond types and phases:
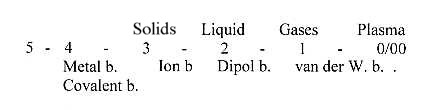
Above
has been pointed to the connection
between bond types and phases: Ion
bonds in salts the solid phase,
dipole bonds with the liquid phase
of water. The van der Waals bonds
could be connected with a gas phase
if "induced dipoles" or
its time-dependant combination of
attraction and repulsion is translated
into the more mechanical collisions
(to and fro) between atoms or molecules
of gases.
Some
more general annotations about chemical
bonds: From the viewpoint
of an atom, the bond to other atoms
can be described as a centre displacement
0 →
00 (becoming a new centre 0'), from
centre of the atom to the centre
of the bond on its circumference
- a virtual place for insertions
from outside.
Overlapping orbitals
(counter directed = of the same
sign) define a new centre. However,
the bonds may be imagined as derivations
from a field level underlying the
atomic level of chemical elements:
a) fragmentation - polarizations,
b) bonds "the other way
around". (Most elements exist
in nature as molecules at lower
temperatures.)
From
this aspect orbital centra could
be regarded as the basic structures
which orientate the atoms. Compare
the precipitation of amino acids
from mixtures of smaller molecules.
Release of energy
at creation of bonds could be interpreted
as (partial) depolarizations - a
return to a higher d-degree. [The
molecule or the chemical compound
could be compared to a linguistic
phrase: The development of an underlying
unit, in languages = the "sense",
regarded as stepwise polarizations
into directions, words for directions,
verbs for actions or processes,
nouns and qualities…] Assuming
a development from fields to mass
to particles representing charge
etc. through polarizations, inversions,
angle steps, then one branch of
the fields may be thought of as
expressed in the coordinate axes
of the atom, as drawings in the
surroundings, in the negative energy
of "vacant space". The
other branch, that from the 00-pole,
imagined as meeting "the other
way around", from outside,
as potential counterdirections ... ...
[The reactive rope ends
of molecules are called radicals,
a word which originates from the
word "root".]
|