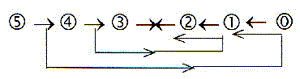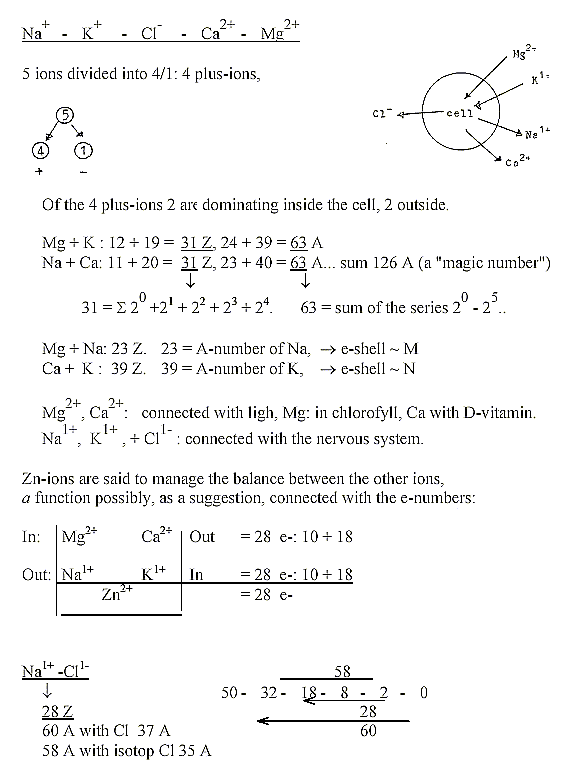|
Application of views from the dimensional mode:l
(With some repetitions from other files.)
See also file The cell, first pages.
There are 5-6 elements primarily which build up the main structures
of life as lipids, carbohydrates,.proteins and nucleic acids. The
number worth noting.
H
- C - N - O - P - S in order
of weight
Valences :
P
- C - N - O/S - H
5
4 3 2
1 as
numbers in a dimension chain.
P, with valence 5 in phosphate groups PO4 (to
~PO3) has a special function as a "binding force" in the
other main substances - for instance in the DNA-spiral and in phospholipides
and as energy storage in ATP. These functions could give arguments
for interpreting the valence number 5 as an expression for dimension
degree (d-degree) 5 as a first binding force. (See further P-groups
below.)
In whch sense can the valence numbers represent d-degrees? Elements
has been regarded as developed in step 2 - 1 in the underlying
fundamental dimension chain, With development of a new dimension
chain in this step (according to the principle of level development
in this model) the different secondary d-degrees could be expressed
linearly, that is in terms of d-degrre 1 - as valences,
a number of potentially bond directions.
(In relation to properties of the fundamental
chain --> Direction (vectors) --> mass/space --> charge
--> distances , it would perhaps be possible to regard the C-atom
as an expression for the vector character in directions (in its
building of C-skeletons), and O-atoms as an expression for charge,
but hardly the N-atoms in any certain sense related to the property
Mass or Space!)
A figure connecting P---O and C---N, with the loop model of the
dimension chain and "outer poles" indicated, here as valences:
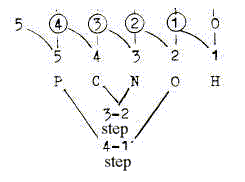
Here the poles represent valences but the d-degrees the polarization
of 5 in 4–1, and 3–2.
In the fundamental chain of the model mass and space
are assumed as d-degree 3 in relation to Charge as a property defined
in d-degree 2. One could here see the uncharged C-atom as the space-building
one, while N and O represent charges, in opposition to one anotheter:
N plus as inward direction, the O-atom the negative charge as outward
direction. So for instance in the peptide bonds (NH3 --- COO-).
Direction around the atoms:
The division of bond directions around the atoms seem to illustrate
dimension steps:
C-atom: bonds 4 →3 + 1, e. g. the
tetrahedron configuration in amino acids.
N-atom bonds: 3 →2 + 1, e. g. in
peptide bonds or when bound in rings of bases.
O-atom bonds: 2 →1 + 1.
(The bond directions become stepwise more polar
in order C → N →
O,)
The P-atom with 4 O-atoms, one oxygen double-bound, crosswise distributed,
seems to illustrate the step 5-4, 4 directions outwards, one complementary
inwards, as illustrating a still unpolarized d-degree.
It ould be noted that the Z-number 15 of P-atom
is the sum of poles in the dimension chain 5 -4 - 3 - 2 - 1.
Classes of substances:
The elements may be regarded as characterizing main classes of substances,
connected
N with proteins (and coding bases),
O with carbohydrates,
H (in CH2) with lipids.
These classes could in some sense on a macro-scale level be regarded
as equivalent with (~) following d-degrees in the cell:
lipids ~ surfaces, 2-dimensional,
carbohydrates ~ volumes, 3-dimensional,
proteins then ~ vectors, 4-dimensional.
Hence, as characterizing macro-molecules, the valence numbers of
the atoms N - O - H as 3 - 2 -1 are increased 1 d-degree.
K-L-M-shells:
The elements H-C-N-O-P-S represent shells K-L-M, shell numbers 1-2-3
in the periodic system, and orbitals 2-1, 1-0/00. Inversely we could
regard these shells - from inside outwards in the atomic structure,
as steps 5-4-3 in the chain of processes.
With debranched degrees meeting "the other
way around, step 5 → 4 gets represented
by the K-hell, step 4 → 3 by the
L-chell; both views are possible.
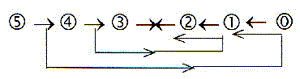
The 2x2-chain behind the periodic system:
5 4 3 2 1
50 — 32 — 18 — 8 — 2 —
0 numbers for additions to whole
shells
P N L K whole
shells
O M
( x) f d p s orbitals,
intervals in numbers of electrons
C-N-O-atoms belong to L-shell and the p-orbital in step 2 - 1,
P and S to the M-shell of higher d-degree..
In the dimnsion model higher d-degrees are defined
as binding forces in relation to lower ones and it could be observed
that P and S in the 3rd shell appear as elements with binding properties
on a higher, more complex level in DNA-RNA (P-atom) and among proteins
(S in the amino acid Cys, creating S-S-bonds in the folding of proteins).
Covalent bonds:
The covalent kind of bonds give the structures in living organisms.
These bonds of the elements imply "shared shortage"
in relation to the "octette rule". Number 8 in the outmost
shell represents a complete surface. Cf. number 8 in the 2x2-chain
at x = 2; in the dimension model d-degree 2 for surfaces.
It's worth pointing out that it's not complementary
poles (atom kinds) that bind to each other here but more or less
similar ones, giving counter-direction mutually (→
←) seen. (The degree of covalent
bond beeing 60-100 %.)
About metal ions, see further below.
Some numbers:
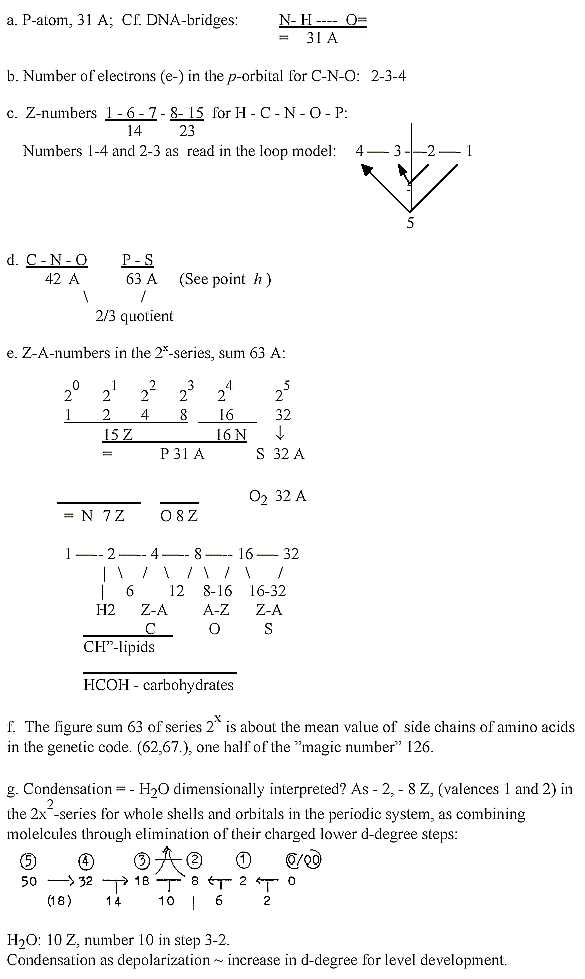
h. "A-Z"-numbers of elements:Number readings
downwards with additions:
95 + 94 = 189, 94 + 74 = 168 etc.:
This "A-Z"-chain may illustrate the two main fusion processes:
the right half the first elementary fusions from e.g. D to He to
Li... (with steps between dismissed), the left half the carbon-nitrogen
cycle in the sun. Here we have factor 21 in the steps and numbers
42 and 63 (point d)
Metal ions as "trace elements":
The function of metal ions is partly not understood (or hasn't
been earlier (1976).
Some general hypothetical aspects are given here departing from
the dimension model:
1. At the level of elements in the periodic system metals may be
interpreted as representing the "00-pole" in relation
the non-metals as "0-poles" (i.e. C-N-O…). This polarity
(complementarity) expressed in minus/ plus, lack versus surplus
in relation to the octet rule.
According to first postulates in the model, metals
then corresponds to "anticenter", inward direction and
polarizing forces.
2. Metal ions represent "anticenter" as the surrounding
of organic cells, in sea water or soil or clay of some kind. And
charge is positive outside of the cell membranes (rest potential).
Metals are used to build skeletons, by unicellular
organisms, and in multi-cellular animals the skeleton was from the
beginning an exo-skeleton (as "surfaces"), which through
immigration (direction inwards) became an endo-skeleton. This illustrates
one general principle of life, the successive built-in of the 00-pole
during evolution (as later in the evolution the built-in of the
environments differentiates internal forces into a psyche).
With regard to the organs, the lighter metal ions Na+
and K+ gets the central function in nervous signals,
Ca2+ role in skeletons and muscles. The nervous system
and skeleton cells derive from the neural plate at the animal pole
of an embryo and ectoderm, in embryos the 00-pole, outer cell layers
(Biology, not yet translated files in this booklet series).
At the same time some metal ions as Fe, Mg, Cu
become built-in to centres in coenzymes, as in porphyrines: centres
as end points of inward directed vectors. (We could perhaps regard
these heavier metal ions from a higher atom shell as stronger vectors,
pointing deeper inwards.) There is also the feature in our model
of a "pole exchange" in last step of the dimension chain,
inward directed motions defining new centres.
(Such a pole exchange may possibly be expressed
as the repeated changes in potential over the membranes of axons
making up the nervous signals.?)
The 00-pole represents also manyfoldness and separation: as primarily
isolated atoms in contrast to the structure building non-metals.
The "S-curve" which describes the gradual transition
from dominating covalent bonds to ion bonds in electronegativity,
may be regarded as an expression for the complementary relation
between non-metals and metals in lower d-degrees: a polarity "concave/convex"
as one of the assumed geometrical definitions in d-degree 2.
Geometrically the inflection points of such a
curve (representing a polarized surface) make up a line, d-degree
1 (2b---1---2a in the model) and could eventually be though of as
defining a first border for a cell. (Cf. lipids.).
It follows from the assumptions in the dimension model that metal
ions as 00-poles should have a polarizing effect or function. The
common expression that many enzymes are "activated" by
Me-ions seems to imply just this fact. A few examples:
- Ca contributes to cell division.
- Fe divides the O2-molecule (in haemoglobin)
through a step Fe2+ →Fe3+.
- Mn takes part in photolysis, the division of
water.
(Very hypothetically one could wonder if for
instance the richness of Zn in eyes - (and of Ca in the balance
organ) has something to do with the "bifurcation" to
2 eyes or 3 semicircular canals?)
Metal ions, incorporated from the environment, go to and gather
in different organs according to their indivual atomic kind. Hence,
there is a connection between one or a few organs and a certain
metal. It doesn't seem as only the electron configuration furthest
out, the group in the periodic system, was determining this connection,
even if this also may be the case. (Ca, Calcium, and Strontium in
the same group go both to bone tissue).
Cadmium, Cd, and Hg belong to the same group but
Cd is said to gather in kidneys while Hg gathers in the nervous
system and brain. Zn in the same period goes chiefly to the eyes.
A general hypothesis here is that mass numbers
like Z-numbers are derived from a dimensional evolution, and also
on a higher level the embryonic development of different organs
in organisms. Then it ought to be possible, at least theoretically,
to trace a dimensional connection between the different Me-ions
and the differentiation of organs?
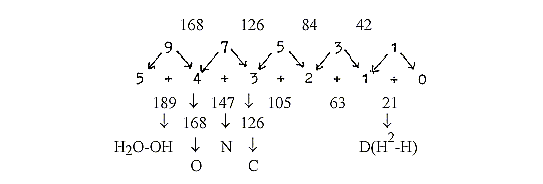
Some small annotations about the 5 ions Na, Mg, Cl, K, Ca:
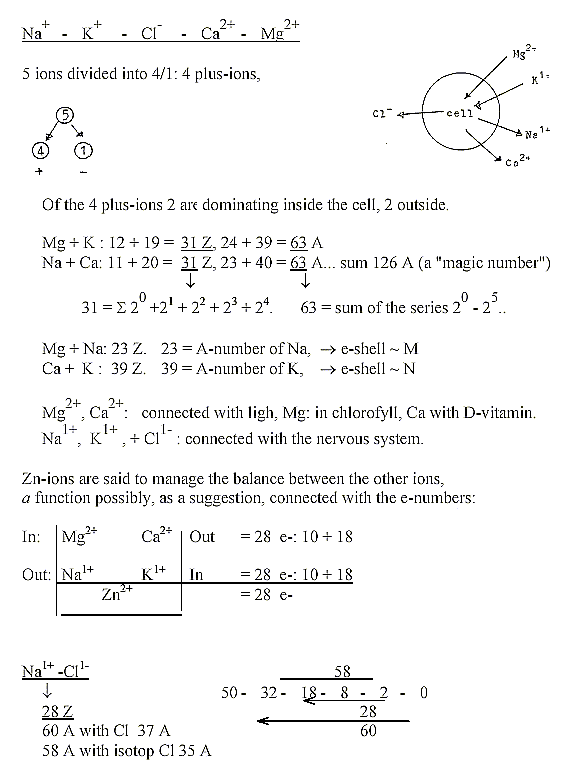
P-groups,
- various small annotations:
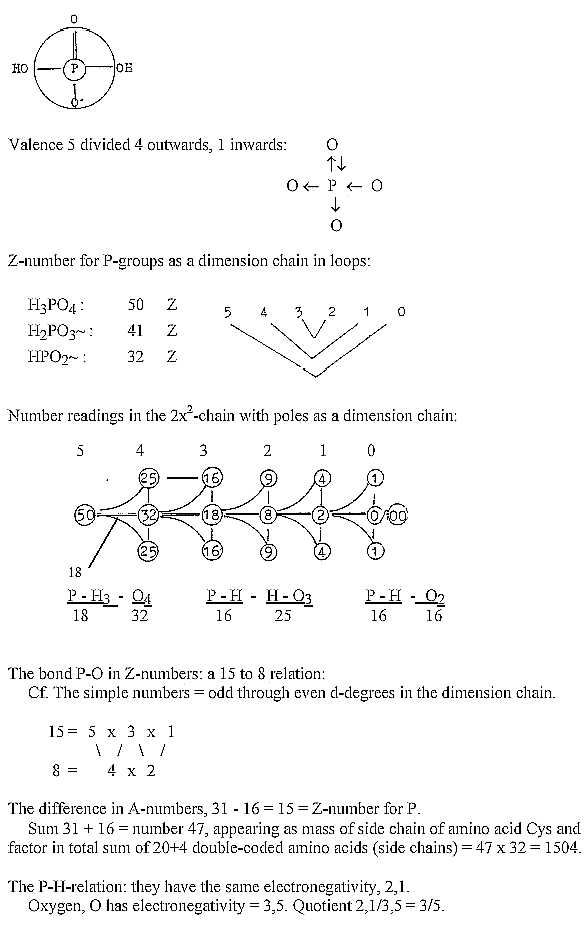
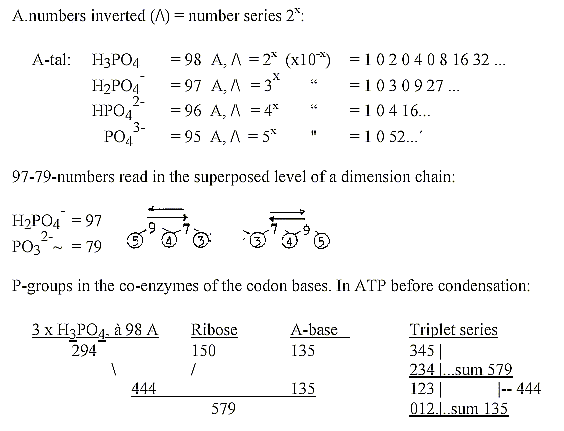
*
|