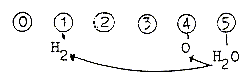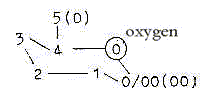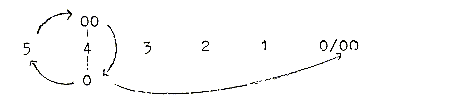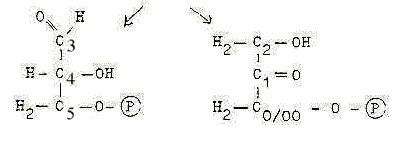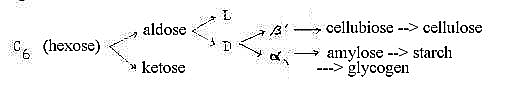|
A very first note: The ring-closing of sugar molecules could reflect
the last step of the carbon-nitrogen cycle of fusion in the sun:
after synthesis of protons from C via N to O back to C with
emittence of an α-partic1e.
Cf. "A-Z"-figure here.
1. The OH-language?
The carbohydrates (cbh) have their own language - the OH-language.
They are individualized not only in number of HCOH-groups but in
different directions of OH-groups from the individual C-atoms. What
does this imply?
It obviously indicates a differentiation in the
configuration of electrons around the separate C-atoms. (Cf. the
sp3- to sp2-hybridization.)
An illustration of two opposite directed dimension
chains could illustrate such a differentiation between electron
shells of C-atoms, in the figure below with one step of displacement.
Of the opposite chains one could be regarded as corresponding to
the d-degrees of structure, the other the d-degrees of motion, or
alternatively just a- and b-poles, as in our primary model.
Mass of the group HCOH is 30 A. That's the sum of poles in a dimension
chain. The number of such groups seem primarily to be 5. as dimension
degreex in our model. (Including 0/00, the "d-degree of motion",
as substantiated, gives number 6.)
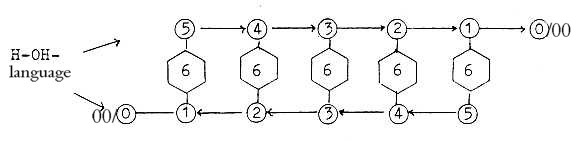
An elementary script of Nature? On the Z-level writing a series
of sexes for the C-atom.
Cf. perhaps that inversion of 15, sum of series
5 - 0, = 0,666...
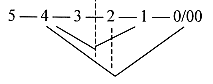
The C-atoms become here individualized in different relations between
the chains. We could attach the numbers 5-4-3-2-1-(0/00) to
the C-atoms in the chain not just as a convention in biochemistry
but as "5/1, 4/2, 3/3, 2/4, 1/5. (Eventually the intervals
as "+4" — "+2"
—0 —
"-2" — "-4"
as differentiating?)
If the script would be so simple, it implies that
the electron shells of C-atoms is dividable in several ways and
may be composed in several ways. s-electrons of both K- and
L-shell should be included — and
engaged in the bonds arround the C-atom.
The varying directions of OH-groups on the surface
of cell membranes function as a language.
2. The incorporation of a 6th C-atom:
What about the hexoses in this imagination?
It should be noted that never more than 5 C-atoms are included
in the ordinary structure-building ring formations of carbohydrates.
It seems that the only way for 6C-atom rings to get formed is through
sharing C-atoms with other rings as in steroids or with open C-chains
as in carotenoides.
The 6th HCOH-group is built in by plants by absorption of the molecule
H2CO3 (or
HCO-). (Minus O2 giives H-COH.).
It starts from ribulose, a pentose, in P-P-bonds.
(The P-groups as connected with 0- and 00-poles in the figure above.)
We may note that the 2 P-groups charged à
39 Z exactly balance the Z-number of this bound ribulose, 78 Z.
The 6th HCOH molecule gets built-in in the middle of the
ribulose chain, more or less at the same time that this chain gets
halved into two C3-pieces (treoses with COO-groups at at ends in
the middle. Through +2 H - H2O the two
C3-pieces unite again to an hexose.
Why in the middle?
Even if we adopt the view on the process as a substantiation of
last d-degree 0/00 in the chain, we have to regard it from the perpendicular
aspect on the chain: the double direction from higher d-degrees
and from lower degrees meeting in step 3-2.
Or with two opposite chains:
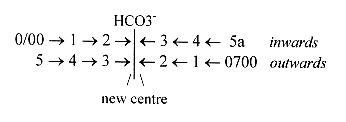
Sums vertically here 5: Only 5 electrons involved
in hexoses ! ?
This is underlined by the 2 P-groups. The central
role of these P-groups, (here as well as in the partition of fructose
at start of glycolysis), can be regarded as polarizing force from
outside as anticentres, initiating the polarization steps - and
also the driving force towards development of a new level through
step 3-2.
The built-in of the 6th C-atom group implies a displacement of
the middle in the chain half a step, a decrease half a d-degree,
as from 3 to step 3 - 2, (from "border to interval",
the elementary illustration of a quantum jump proposed in files
about Physics).
We could compare with transformations between number base systems
(nb-x),
from 10 to 6:
nb-10: 12 = C, 18 = H2O
(Mass
numbers A)
↓ ↓
nb-6: 20 30
= HCOH A quotient and a relation in step
3 - 2.
The A-numbers 12 and 18 as intervals:
nb-16 —— nb 10 —— nb 8:
32 —|→ 50 —|→ 62
(= H2CO3,
- O2, 32)
18 12
In the Pentosephosphate cycle, where 5 C3 get tranaformed into
3 C5.molecules, one C3 gets a C2 from C4, giving C5, another C3
gets C2 from C7, giving 2 C5.
(There are also in intermediate steps, C6 - C4
and C7 - C3 molecules, reminding of the number 10
division chain.)
3. Elementary numbers of carbohydrates:
Some simple associations with the dimension chain in alternative
forms.
- Numbers O = 8 Z, H2 = 2 Z, a relation 4 —
1.
- Why primarily pentoses as a condition for synthesis of carbohydrates?
150 A is 5 times 30, the sum of poles of the dimension
chain.
E-numbers = value as sum of outer poles in the different d-degrees.
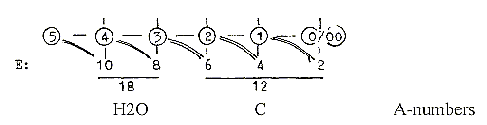
Numbers of the carbohydrates in the 2x2-chain:
All numbers Z.
H2CO3,
the molecule which (minus O2) is built-in as the 6th HCOH-molecule.
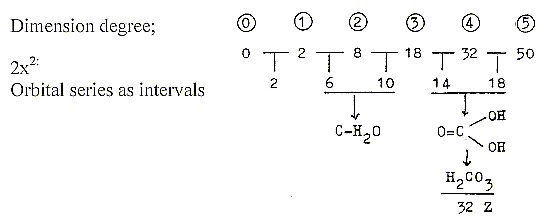
Mass numbers with 2 chains 2x:
Hypothetically the 2x-series is assumed as valid in polarizing
direction inwards in a dimension chain.
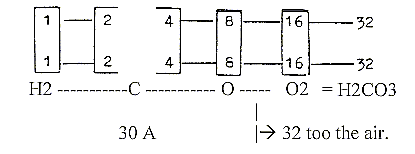
The division of H2O:
a. Elementary dimension chain: Z/2:
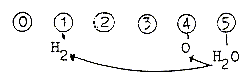
b. 2x-chain, A-numbers:
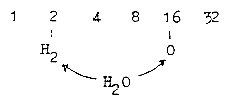
c. 2x2-chain:
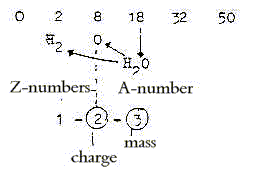
4. The ring-closing of carbohydrates:
A first question: Why do the ends differ in open, unclosed carbohydrate
chains? One end is the aldehyde group H-C=O and the other end H2-C-OH.
It could be read (-/+), minus H at one end (aldehyde
group) and plus H at the other. It's suggested here that this reflects
the polarization in last step of the dimension chain from d-degree
1 to d-degree 0/00,
1a —
0/00 — 1b,
Sum of poles = "E-number" = 2 for 2 H, poles which define
a (new) 0-pole and 00-pole respectively, here identified with minus/plus
one H.
With C-atoms regarded as representing different d-degrees,
the closing to rings of pentoses or hexoses may be interpreted as
illustrating the connection between these outer poles 0 and 00 (also
together representing 5').
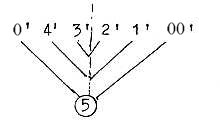
Conventionally the numbering of C-atoms begin with
C of aldehyde group as number 1. Accepting this order but decreasing
the number series one step, relating it to the dimension model gives
in an hexose:
H2COH —
HCOH — OHCH —
HCOH — HCOH —
O=CH
5 4
3
2 1 0/00
(~5')
Then, in terms of the dimension model, the ring-closing
implies a connection from d-degree 4, outer poles of which are 0
and 00, with the C-atom at the end representing d-degree 0/00 where
these poles are met again.
00
|
4 — ———
0/00
|
0
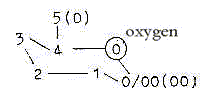
Perhaps the oxygen atom of the aldehyde group could be identified
as expression for the 00-pole "meeting the other way around".
Within P-P-bonds, as for instance when C6 glucose is transformed
to C6 fructose at start of the glycolysis, the oxygen bond changes
to a relation 4 — 1:
C4 —Oxygen —
C1:
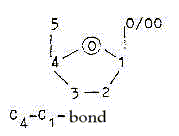
The same figure can illustrate pentoses with only an OH-group at
position 0/00.
The C4-C1-connection implies an angle change, similar to the turn
from a more linear aspect on the dimension chain, formally 180°,
to the loop model, 90°, a vertical aspect on polarizations:
5 → 0/00, 5 → 4/1,
5 → 3/2:
The transformation of glucose to fructose implies
that the middle of the rings is increased half a degree, as it was
decreased a half degree when the 6th HCOH-group were built in, here
from 2 in glucose to step 3-2 (C3 —
C2) in fructose (as in pentoses).
(We may here remind of the two classes of amino
acyl tRNA-synthetases related to C3 and C2 in ribose of nucleotides
at the protein synthesis, dividing the attributed amino acids
into 2 groups, as the opposite directions of the chain meet in
this step 3-><--2.)
In the dimension model d-degree 3 gets polarized in poles 3a—3b
(defining d-degree 2). We suggest to compare with the more or less
immediate division of a hexose when the 6th C-group has been built-in,
into 2 C3-pieces - and the same division in this 3-2-step when glucose
has been transformed to fructose at start of glycolysis.
Closing of the carbohydrates into rings is geometrically, in the
macro-structure, expressions for the steps from d-degree 4 →3 →3a in our dimension model.
The model implies also that rotation as a 2-dimensional motion appears
in step 4 →3. Also assumed as an
angle step as 180° to 90°.
Such a rotation appears also here at closing of
the ring around C4: H turns 180°, OH-group 90°, which also
leads to the turn of C5 90°. The OH-group is turned towards
C "0/00". Hence, ring-closing and rotation seem connected
as geometry and d-degree of motion in the dimension model.
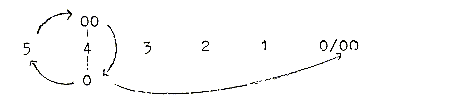
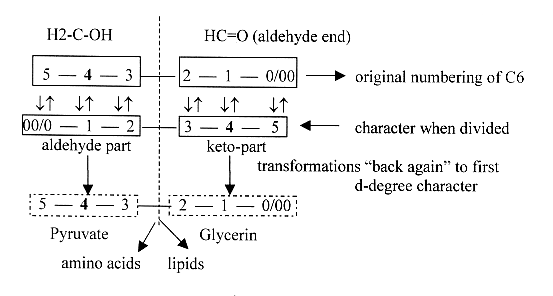
5. Aldehyde - Keto parts:
When hexoses as fructose are split into two C3-parts, one gets
an aldehyde end, the other a keto-bond in the middle: we have an
aldehyde and a keto-part.

Original aldehyde end to the right at C0/00
C3 — C2
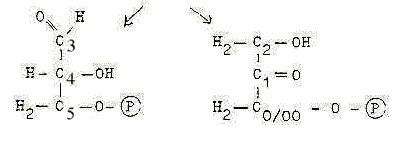
- Original ends COH and H2COH
now appears at C3 and C2. (Equivalent with minus 1 H at C3, +1 H
at C2). The part with original aldehyde end becomes the keto-part.
A change in direction of numbering seems needed if the keto-group
C=O should represent d-degree 4 in the C-chain.
- The main features in the process of glycolysis and
citrate cycle may in some respects be regarded as an illustration
of the double direction in a dimension chain:
5 → 4 →
3 →<— 2 <—
1←0/00
3C
+ 3
C
The halving of the fructose molecule as a polarization outwards,
one half in d-degree 3 meeting +1 C + 2 C (CO2
and Acetyl~) as from the other half, representing the complementary
pole to isocitrate 6 C.
- It's the aldehyde part C3, here numbered
5 - 4 - 3, which develops through up to ten steps to C3 Pyruvate
through glycolysis, and further in synthesizing direction into
citrate cycle, + C1 and + C2 to C6, isocitrate, that's to the higher
d-degrees of C-atoms.
The way is connected with generation of amino
acids and proteins.
- It's the Keto- part, here numbered 2 - 1
- 0, with the double-bound oxygen at C1, which develops to C3 Glycerine,
the "circular" backbone part of membranes.
- The separate ways of transformations for the C3-pieces
underscores the differentiation of C-atoms in glucose as well as
the geometrical polarity "radial" versus "circular"
(3b-/3a-poles) as amino acids (proteins) versus cell membranes on
a macro-scale.
From Pyruvate the way leads also to C2, Acetyl~,
which starts the synthesis of the radial parts of membranes, the
fatty acids.
If we want to associate higher C-numbers 3-4-5 as
C-numbers with the way to amino acids, the lower ones with the way
to glycerine, according to dimensional aspects, and further double-bound
oxygen with d-degree 4 as C4, the figure below could illustrate
the ambiguousness in numbering and transformations (as "pole
exchanges") between the two opposite number series.
A division vertically of the horizontal chains
shows that both C3-parts "virtually" contain whole dimension
chains. Cf. in the model debranched d-degrees 1 --- 2 from higher
steps meeting the other way around.
Identifying C4 with d-degree 4 (in the model double-direction)
may give an aspect on why transformations always have the direction
from keto-form to aldehyde form (possible to read as outwards?)
and why the keto-form form is the actual one when carbohydrates
transform into one another.
The association of C-atoms with different d-degrees seems supported
by the change in mass distribution during glycolysis: The transformation
of the aldehyde part as 3-P-glycerate to Pyruvate in several steps
implies that a nearly equal mass 30 - 30 - 30 transforms to 45 -
28- 15 in Pyruvate, CH3— C=O—COO(H), that's quotients
circa 3 - 2 - 1. as if an underlying differentiating process between
the C-atoms manifested itself. (In reality expressed as plus / minus
16, oxygen, between first and third C-atoms.)
6. Polarizations of the OH-language:
Different directions of the OH-groups around the C-atoms seem
possible to regard as a way through polarization steps, different
paths at bifurcations leading to different roles and positions in
the cell:
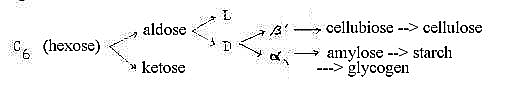
The figure shows three such polarizations where the 3rd leads to
the opposition between cellulose <—> amylose and starch:
it implies one kind of 2-3-relation in d-degrees regarding
the forms on a macro-scale: cellulose for cell coats, surfaces,
amylose as substrate in the cell, volumes. (Cf. different coenzymes
connected with different carbohydrates: UTP with glucose, TTP with
cellulose.)
This 3rd polarization, α,
β, refers to the orientation of the
OH-group at what here has been regarded as C-atom "0/00"
in original hexose.
A 4th polarization in hexoses in direction "up/down"
at C3 (conventionally C4) gives the difference galactose/glucose:
united in the disaccharide lactose.
We have hypothezised that the 2x series may reign, from
the end of a dimension chain and (formally) there are 25
isomers in a hexose, C6, 24 in a pentose, 23in
a C4 piece, 22 in a C3-piece:
Carbohydrates: C3 C4
C5 C6
Number of isomers: 4 8
16
32
Following figure may not illustrate the real way of derivations
but could perhaps give a hint of the way of thinking here, with
associations to the dimension model:
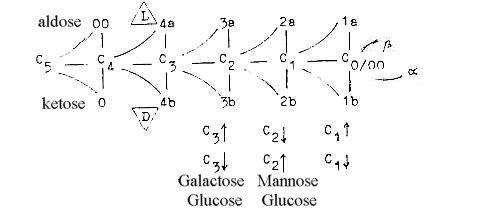
- Arrows for the direction of OH-groups.
- The β-form should be associated with
the 0-pole and pole 4b (D), (outward direction).
- The up/down polarity of OH-groups could perhaps be expression
for the polarization at C1, represented in each step.
7. Forms of macro-molecules of the carbohydrates:
Another aspect on the different carbohydrates gives the behaviour
of the polymerized macro-molecules if we regard them in terms of
motions of different dimension degrees:
- Cellulose gets folded, which may be seen as an 1-dimensional
motion to and fro, a kind of vibration.
- Amylose gets spiralled, a kind of 2-dimensional rotation,
connected with pathway motion giving a 2- to 3-dimensional motional
structure.
- Amylopectin and glycogen get branched, as illustrating "translation
in 3 dimensions".
In this respect there is no opposition between d-degree of form
(structure) and d-degree of motions; the motions if we regard the
formations of the macromolecules as such, are and give the macro-forms.
8. Some number operations;

*
|