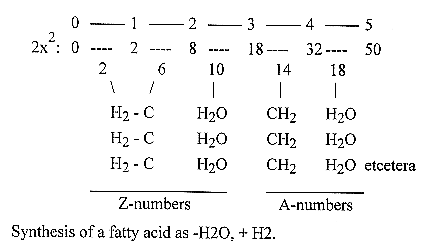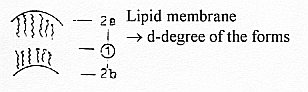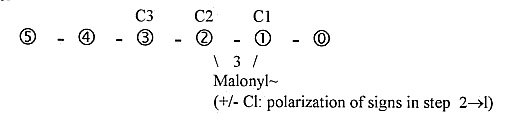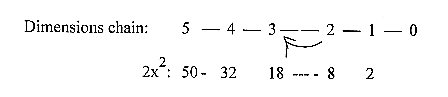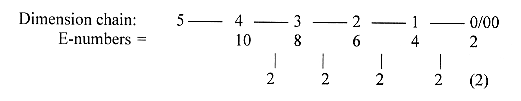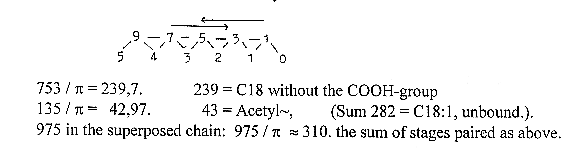|
Some general
aspects:
1.
Main feature of the process
from carbohydrates to fatty acids
implies the excluding of oxygen
while storing H2.
One suggestion below is to think
of this process, the creation of
enclosing barriers, as a result
of steps toward lower degrees in
a a dimension chain.
The
aspect on elementary atomic masses
gives the order O-N-C from higher
to lower mass (cf. the opposite
direction in the carbon-nitrogen
cycle in the sun, carbon →
nitrogen →
oxygen , minus alpha, back to carbon,
and "A-Z-numbers").
The excluding
of water, A- and Z-numbers in the
process: numbers in the 2x2-series:
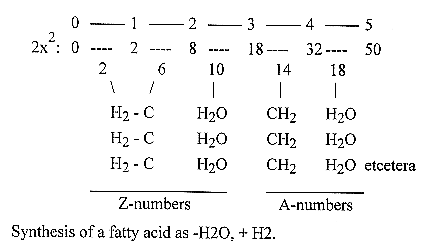
(Mass a property in d-degree 3 in
relation to Charge as a property
assumed in the model as defined
in d-degree 2.)
2.
Fatty acids, bound to Glycerine
(Glycerol), build the cell membranes,
(together with P-groups,
P for phosphorus). The macrostructure
of a membrane may illustrate d-degree
2 and 1.
Glycerine
as "the backs",
-
binding 2-3 fatty acids as 1-dimensional
zigzag chains turned toward one
another,
inside-outside
as opposite poles: 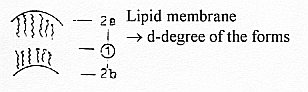
Fatty
acids with glycerine build the barriers
to a water environment, that's surfaces
and as such 2-dimensional on a macro-scale.
The same character is reflected
in the synthesis of the fatty acids,
if we may regard the number of C-atoms
as expressions for d-degrees, roughly
an addition of C2-pieces.
In
the loop model of our dimension
chain 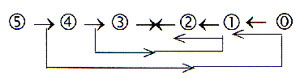
the debranched degrees from steps
5 - 4 - 3 are meeting the other
way around; the d-degree step 4→ 3 correspond to d-degree
step 2 ←1 and 3→2 to a kind of half
step back 3 ←
2.
At
the same time (compare step 4 →
3 in the figure) the fatty acids
as "linear" have the character
of 4-dimensional vectors in opposite
directions. The membranes with glycerine,
illustrate also the geometrical
poles 3b versus 3a of d-degree 3,
radial versus circular form. The
division of C6 into to halves C3
reflect the polarization of the
complementary character: one half,
Glycerine C3, as forming part of
the circular structure, with feature
from the 00-pole (ac) and inward
direction, and one half further
transformed outwards during glycolysis
to Pyruvate C3, from which one branch
leads to Acetyl~, C2, and the synthesis
of fatty acids, the radial part
of membranes, with feature from
the 0-pole (c) and outward direction.
In number of C-atoms:
Glycerine versus
Malonyl~ (from Pyruvate) as 3a---3b-poles
in d-degree 2: 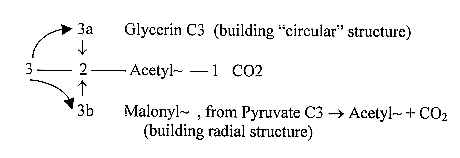
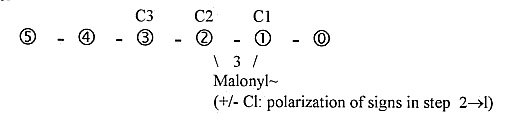
It could be
noted that Pyruvate represent the
border to the citrate cycle, and
the glycolysis in relation to this
cycle as whole processes have also
the geometrical character of radial
versus circular.
The
bifurcation of the way for Pyruvate,
into the citrate cycle with + C1
in direction of synthesis to keto
acids and amino acids, and outwards
to Acetyl~, C2, to fatty acids,
represent a main division in classes
of substances and their role in
cells, with similar geometrical
complementarity on that level.
The fact that
Acetyl~ also enters the citrate
cycle (with an OH-group) after a
couple of steps (forming isocitrate)
seems as an obvious example of the
views in the loop model above. The
transformation from Pyruvate to
Malonyl~ implies that the COO-group
is moved to the other end of the
C3-chain:
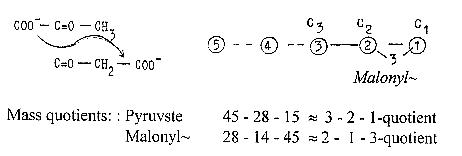
Pyruvate and Malonyl~
uncharged. This displacement
may be regarded as an essential
expression for the change of direction
in the loop model above, here from
outward fragmentation direction
toward the synthesizing inward one
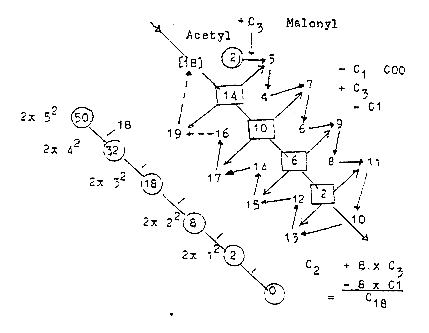
. 3. The synthesis of fatty
acids in detail:
It starts
with C2, Acetyl~ (CH3-C=O ~).
Another Acetyl~
C2, +C1 forms Malonyl~, C3 (COO-CH"-C=O
¨) , which is attached to another
site and the first C2 (Acetyl~)
moves to bind with this, debranching
the C1- (COO-group) of Malonyl~.
Compare the dimension chain:
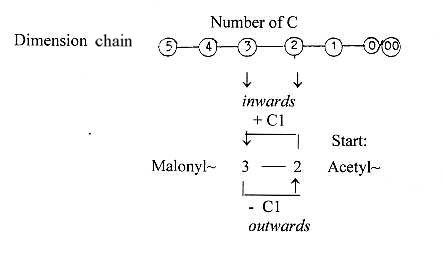
Since
the "outer poles" (or
partial structures) of d-degree
2 and 1 is 3a/3b and 2a/2b, the
illustration of the process could
be imagined as moved to step 2-1
and connected with the a-poles for
instance. (+/-
Cl: polarization of signs +/- in
step 2-1 as a parallel to
the
polarization of charges in p/e on
the atomic level?). In
the dimension model d-degree 3 have
the "outer poles" 4a ---
4b, d-degree 2 the poles 3a -- 3b.
We may note
- as a coincidence? - the mass numbers
of Acetyl~ and Malonyl~ :
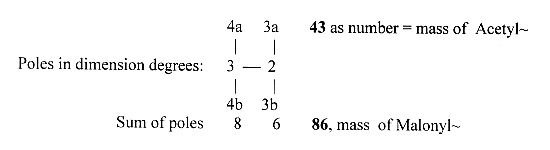
*86 = Malonyl~ charged: C=O —
CH2 — COO-~
4.
The synthesis as a process of repetitions:
The synthesis of the fatty acid chains: /\/\/\/\/\/\/\/\/COOH
is a process between Acetyl~ (C2)
and Malonyl~ (C3) with removal of
C1.
Keeping
to the vector character of d-degree
4 in fatty acids, the multi-enzyme
complex with two S-binding sites
where the synthesis takes place
could illustrate an oscillation
between poles 4a and 3a.
Connecting
the dimension chain with angle steps
in a circle, the division to d-degree
4 implies a division to 180°,
in next polarization a division
to 90° - according to our first
assumptions in the model. Positions
at 180° and 90° seem possible
to connect to the S-binding sites. The
multi-enzyme complex as structure
giving a picture of angle steps
through a circle: 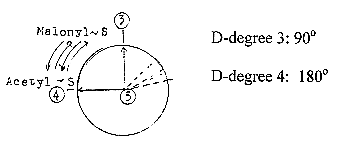
a.
Acetyl~ , (C2), gets bound to site
4, S in the R-chain of Cys (47-1
A).
b. Malonyl~, (C3),
gets bound to site 3, S in Pantetheine,
(358 -1 A), part of HS-CoA
c. Acetyl~ connects to C number
2 in Malonyl~ at site 3, and the
COO-group of Malonyl~
is debranched.
This combination
C2 + C3-piece gives virtually a
C5 piece, immediately divided
C5 →C4 - C1: cf. first step
in the dimension model with 1 d-degree
debranched.
d. The C4-molecule
gets moved back to site 4 (3 →
4) and
e. A new Malonyl~
gets bound to site 3. Etceteras. It's
Acetyl~ that moves "outwards"
to site "3" and as 2 x
C2 inwards again to site "4";
cf. the vector character of directions,
while Malonyl~ (from C2 + C1) always
takes position at site 3. The "vector"
growing through substrate from lower
d-degrees ("anticentre"). The
COO-group of last Malonyl~ becomes
the "head" of the fatty
acid.
(It seems logical then
that the coupling with glycerine
follows.)
Hence,
the synthesis starts from the end
of the fatty acids, which become
double directed towards one another
in membranes, and this agrees with
the interpretation of sites in d-degree
terms, d-degree 4 within membranes
in the double membranes. Outer
poles in step 3 - 2 = 4a/b - 3a/b.
In the 2x2-chain (50
- 32 - 18 - 8 - 2 - 0) the step
4 - 3 corresponds to interval number
14, the CH2-pieces, and 2 + 8 +
18 = 28, the CH2-CH2-pieces.
5. Why are C18 and C16 most common
fatty acids, 8 or 9 C2-pieces?
Most common fatty acids in
animal life:
33
% palmitic acid, C16, saturated:
C15H31COOH = 256 A
17
% stearic acid, saturated, C18:
C17H35COOH = 284 A
35
% oleic acid, unsaturated, C18:1:
C17H33COOH = 282 A
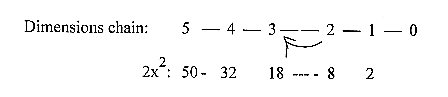
One
aspect: The process towards C18
implies C2 + 8 x C3 (Malonyl) =
26 C = 18 + 8, minus debranched
8 C1, rest C18. (?)
Or:
one pole of d-degree 4, represented
by number 32 in this chain, = 16.
(?) Another answer to the
question could eventually be connected
with the "E-numbers",
the sum of poles in the dimension
chain - if the difference of 2 in
the steps here may represent C2-pieces.
The process as occurring in step
4 - 3, including all lower degrees,
as double-directed to and fro (0
→
1 →2
→3
→
4 →3
→2
→1
→
00), gives 2-4-6-8-10-12-14-16-(18),
counting from d-degree 0/00 with
E-number 2. 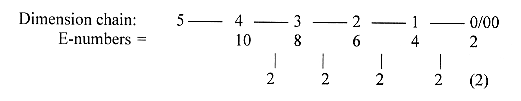
The
fact that double-bonds as in C18:1
appear in the middle of the CH2-chain
could in that case depend on the
d-degree 4 representing double-direction. An
alternative could be departing from
the intervals in the 2x2-chain,
reading Cn-numbers to and fro as
with these intervals: Also here
double bonds appear in the middle:
Number of C in the phases:
2---5---4---7---6---9---8---11---10--13--12--15--14--17-16--19--18:
In steroid figurations C16
and C18 = C24, minus C8 or C6 respectively,
one gets following structures: 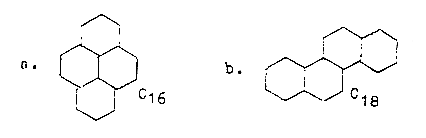
6. Some numbers:
The process in detail with
mass numbers, addition of one C2-piece
(CH2-CH2) = 5 steps:
43 +
86 -
44 +
2 -18
+2
= 71
Acetyl~
Malonyl~ -CO2
+ 2H -H2O
+2H Mass
sum of 9 stages of synthesis
to a fatty acid C18, (bound, without
end-group OH): 
Z-number
for 9 stages, with +1 for bond to
S:
24 - 40 - 56 - 72 - 88 -
104 - 120 - 136 - 152 = 4,5 x
176 = 792, couples added
in the same way. (Cf. number 176,
½ x 352, middle 3-figure
number in the chain below.)
Sum 792 = 18 x
44 is interval between the triplet
series outwards and inwards of the
elementary chain 5 - 0: (543 + 432
+ 321 + 210) - (012 + 123 + 234
+ 345) = 792.
π-number
involved?
π
connected with the geometry of "spherical"
membranes?
The superposed
chain to the elementary one:
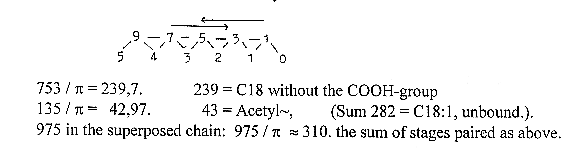 Some
mass numbers of triglycerides:
Maximum with 3 C18: 890
A
Minimum with 3 C16: 806
A
2 C18, 1 C18:1: 888
A
1 C18:1, 2 C16; 832
A With P-group (uncharged)
and only 2 fatty acids: 2
C18: 704
A
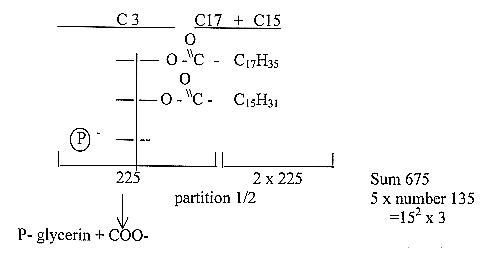
1 C18, 1 C16: 676
A (18+8)2, middle numbers
18 + 8 in the 2x2-chain
P-lipids:
A P-lipid charged, 675 = 3 x 225
(≈
2/3 x the sum of the exponent series
1011 (see about amino
acids.)
Number 273:
Mean value for
the 3 fatty acids, C16, C18, C18:1
if charged = 273 A.
Compare
mean value for 2 unbound amino acids
= 273 A.
Transformations
between number-base systems (nb-x):
256
in nb-10 = 1104 in nb-6 =
3 x 368.
284
in nb-10 = 1152 in nb-6 =
3 x 386
2(368-1) = 734
and 2(384 +1) = 770: this is the
sum of the two codon type groups
of amino acids (side-chains). (Amino
acids)
Z-sums
for fatty acids = mass of G- and
A-bases in the genetic code:
Z-number
for stearic acid C18 = 151, the
mass number of the G-base in codons.
Z-number for palmitic
acid C16 = 135, the mass number
of the A-base in codons.
Tha's when the fatty acids are bound,
i. g. minus OH in the COOH-groups.
Numbers
from a dimension chain, similar
to the Z-A-numbers of Uranium:
Glycerine and a fatty acid C16:
C16 - OH = 256 - 17 A = 239 A
Glycerine - H = 91 A Cf.
triplets in the elementary number
chain inverted:
92 x 2 /\ = 543,47 x 10-5
238 x 2 /\ = 210,08 x 10-5.
238
and 92 the A- and Z-numbers of Uranium. * Continuation; 1/7
- Fatty acids, part 2, collagen
and other substances
|